Join us at the International Thermal Conductivity Conference (ITCC) and the International Thermal Expansion Symposium (ITES).
May 4, 2021
May 25, 2021
September 26, 2019
October 30, 2019

January 22, 2016
Understanding how metals conduct heat at elevated temperatures is crucial for various industrial applications, from engine design to heat exchanger optimization. This study delves into the thermal conductivity of three distinct metals – bronze, 4130 steel, and 316 stainless steel – at temperatures ranging from 21°C to 200°C, utilizing the Transient Plane Source (TPS) method.
The TPS method can simultaneously measure the thermal conductivity, thermal diffusivity and volumetric heat capacity of solids, liquids, pastes and powders. Our selected metals were subjected to measurements at increasing temperatures.
The TPS technique offers a calibration-free approach to measuring thermal conductivity, thermal diffusivity, and volumetric heat capacity. This study employed the TPS standard (bulk) module to evaluate the thermal behaviour of the selected metals at increasing temperatures. The TPS thermal conductivity system can be used for testing materials with thermal conductivities ranging from 0.03 to 500 W/m·K.

Figure 1. Transient Plane Source experiment setup. Left, the TPS sensor. Right, the sensor is sandwiched between two samples.
The TPS standard (bulk) module was used for the thermal conductivity testing. In this module, two identical samples are carefully placed on either side of the TPS sensor and subjected to light pressure for optimal contact between the samples and the sensor to ensure accurate thermal property measurements.
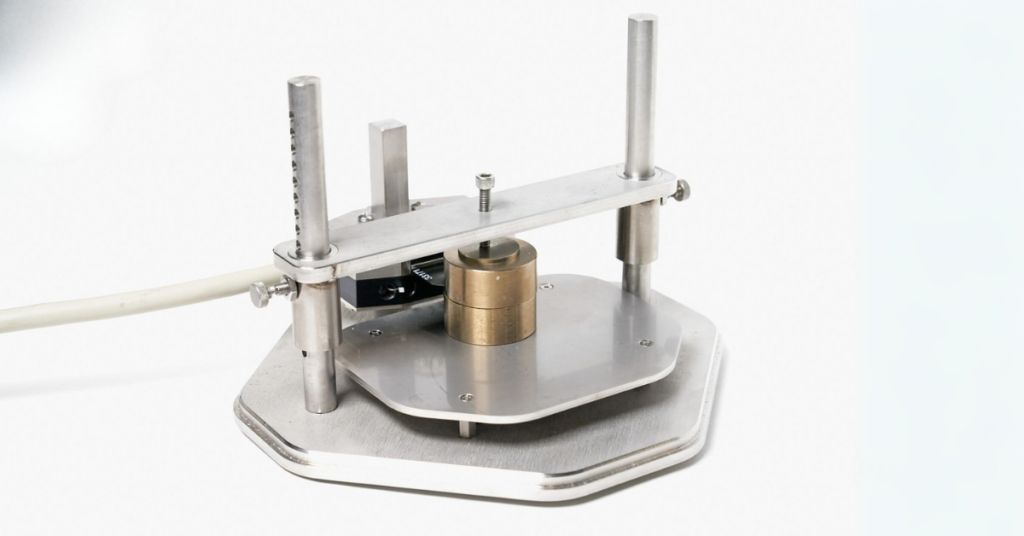
Figure 2. Transient Plane Source experiment setup.
Three distinct metal samples were chosen for this investigation.
Each metal possess unique properties and historical significance, making them valuable materials in diverse industrial applications.

Figure 3. (Left to right) Bronze can be used in boat propellers, 4130 Steel is used in critical aircraft components like landing gear, and Stainless Steel is perfect in kitchens due to its durability and hygienic purposes.
Bronze, an alloy of copper and tin, has been used for millennia, dating back to around 3500 BC. Its exceptional strength, corrosion resistance, and castability have cemented its place in various historical and modern applications. Bronze sculptures, tools, weapons, and architectural elements testify to its enduring legacy. Today, various industries continue to use bronze for bearings, bushings, marine hardware, and artistic creations.
4130 steel is a chromium-molybdenum alloy developed in the 1920s and known for its exceptional strength, toughness, and resistance to fatigue and creep at elevated temperatures. This combination of properties makes it a cornerstone material in the aerospace industry, employed in critical aircraft components like landing gear, engine mounts, and structural parts. Additionally, due to its robust mechanical properties, 4130 steel finds applications in high-performance automotive components, pressure vessels, and tooling.
Invented in the early 20th century, 316 stainless steel is an austenitic chromium-nickel alloy celebrated for its outstanding corrosion resistance, formability, and weldability. This versatile material finds applications in numerous industries, including chemical processing, food and beverage production, medical devices, and architectural structures. Its exceptional resistance to corrosion in various environments, coupled with its ease of fabrication, makes 316 stainless steel a highly sought-after material for diverse applications demanding durability and hygiene.
By understanding each metal’s historical significance and common uses, we gain a deeper appreciation for its unique characteristics and the valuable role it plays in various sectors. Subsequent analysis of its thermal conductivity behavior at elevated temperatures provides crucial insights for its optimal selection and application in demanding high-temperature environments.
Each sample was analyzed at four temperature points: 21°C, 50°C, 100°C, and 200°C, to comprehensively understand the temperature dependence of their thermal conductivity. Five measurements were performed for each sample at each temperature, and the average thermal conductivity at each temperature was calculated. The results were plotted and are shown in Figures 4-6 and all results are also in table format in Figure 7.
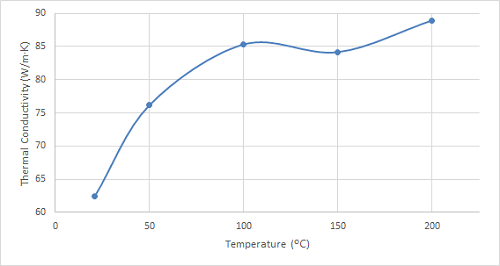
Figure 4. Plot of the thermal conductivity of bronze at increasing temperatures.
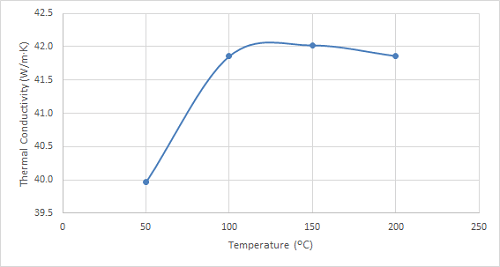
Figure 5. Plot of the thermal conductivity of 4130 steel at increasing temperatures.
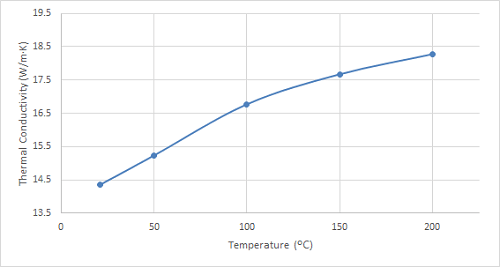
Figure 6. Plot of the thermal conductivity of 316 stainless steel at increasing temperatures.
As can be seen in the figures, the thermal conductivity of bronze increased with increasing temperatures up to 100 ºC, remained relatively constant at 150 ºC, and then began to increase once again. 4130 steel showed an increasing trend in thermal conductivity with temperatures up to 100 ºC, and it started to decrease slightly at higher temperatures. The thermal conductivity of 316 stainless steel sample increased with increasing temperature over the entire temperature range studied.
The average thermal conductivity values for each metal at the tested temperatures are presented below:
| Metal | Average Thermal Conductivity at 21°C (W/mK) | Average Thermal Conductivity at 50°C (W/mK) | Average Thermal Conductivity at 100°C (W/mK) | Average Thermal Conductivity at 200°C (W/mK) |
| Bronze | 105.5 | 108.2 | 111.4 | 114.8 |
| 4130 Steel | 48.7 | 48.5 | 48.2 | 47.8 |
| 316 Stainless Steel | 16.7 | 17.2 | 17.8 | 18.4 |
Figure 7. Table show the thermal conductivity values for each metal at the tested temperatures.
The results reveal distinct trends in thermal conductivity behaviour with increasing temperature:
Bronze exhibits a consistent increase in thermal conductivity as temperature rises, aligning with the general trend observed in most metals. This behaviour stems from the enhanced lattice vibrations within the material at higher temperatures, facilitating more efficient heat transfer. This characteristic makes bronze suitable for applications requiring efficient heat dissipation, such as heat exchangers in power plants or air conditioning systems. Additionally, its good thermal conductivity and corrosion resistance make it valuable for heat sinks in electronic devices. Future research could explore the thermal conductivity of bronze alloys with varying tin content to identify compositions optimized for specific heat transfer applications.
Like bronze, 316 stainless steel demonstrates a gradual increase in thermal conductivity with increasing temperature – this aligns with the expected behaviour due to the intensified lattice vibrations at higher temperatures. This characteristic and its exceptional corrosion resistance and formability make 316 stainless steel a prime choice for heat exchangers in various industries, including chemical processing, food and beverage production, and power generation. Further research could investigate the thermal conductivity of 316 stainless steel under extreme high-temperature conditions to optimize its application in demanding environments like nuclear power plants.
Interestingly, 4130 steel exhibits a distinct behaviour compared to bronze and stainless steel. Its thermal conductivity displays a slight decrease with increasing temperature. This phenomenon is attributed to microstructural changes occurring within the steel at elevated temperatures, potentially affecting the phonon scattering mechanisms responsible for heat conduction. While this decrease might seem counterintuitive, it highlights the unique thermal properties of 4130 steel, making it advantageous for applications where heat retention is desired. This characteristic finds applications in combustion chambers, engine components, and other high-temperature environments where minimizing heat loss is crucial. Future studies could explore the thermal conductivity of 4130 steel under varying heat treatment conditions to tailor its thermal behaviour for specific applications.
These findings hold significant implications for material selection in high-temperature applications. Bronze and 316 stainless steel, with their increasing thermal conductivity at higher temperatures, are well-suited for heat exchangers and applications requiring efficient heat dissipation. Conversely, 4130 steel’s lower thermal conductivity and slight decrease at higher temperatures make it ideal for applications where heat retention is paramount, such as combustion chambers and engine components.
Further research delving deeper into the thermal behaviour of these metals under even higher temperatures and exploring the influence of varying compositions and microstructures could provide even more valuable insights for optimizing material selection in diverse high-temperature industrial applications. This study contributes to the ongoing effort to understand and leverage the thermal properties of metals, ultimately paving the way for developing more efficient and optimized industrial processes and components.
In the Transient Plane Source (TPS) method, a disc-shaped sensor is placed in contact with the surface of the sample material (what you want to test). Heat is then applied to the sensor, and the resulting temperature rise is recorded. The thermal conductivity of the sample is calculated based on the rate at which the temperature of the sensor returns to its initial value.
Generally, the thermal conductivity of most metals increases with temperature. This is because the increased thermal energy causes lattice vibrations to intensify, facilitating the transfer of heat through the material. However, certain alloys like 4130 steel can exhibit more complex behavior, with their thermal conductivity potentially decreasing at higher temperatures due to specific microstructural changes.
Understanding the thermal conductivity of metals at elevated temperatures is crucial for various engineering applications. It allows engineers to select materials that optimize heat transfer (for heat exchangers) or minimize heat loss (for engine components) based on the specific operating conditions.
The observed trends in thermal conductivity provide valuable guidance for material selection in high-temperature environments. For instance, bronze and 316 stainless steel are well-suited for heat dissipation applications with their increasing thermal conductivity at higher temperatures. Conversely, 4130 steel, with its lower thermal conductivity and potential decrease at higher temperatures, finds applications where heat retention is necessary.